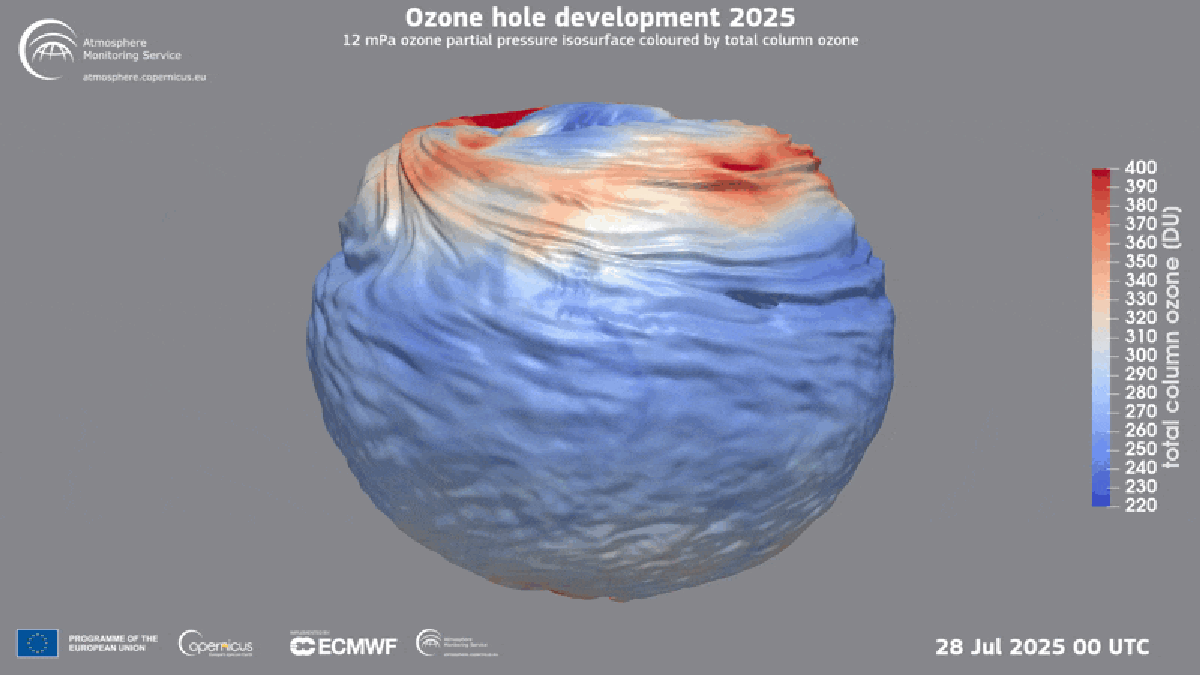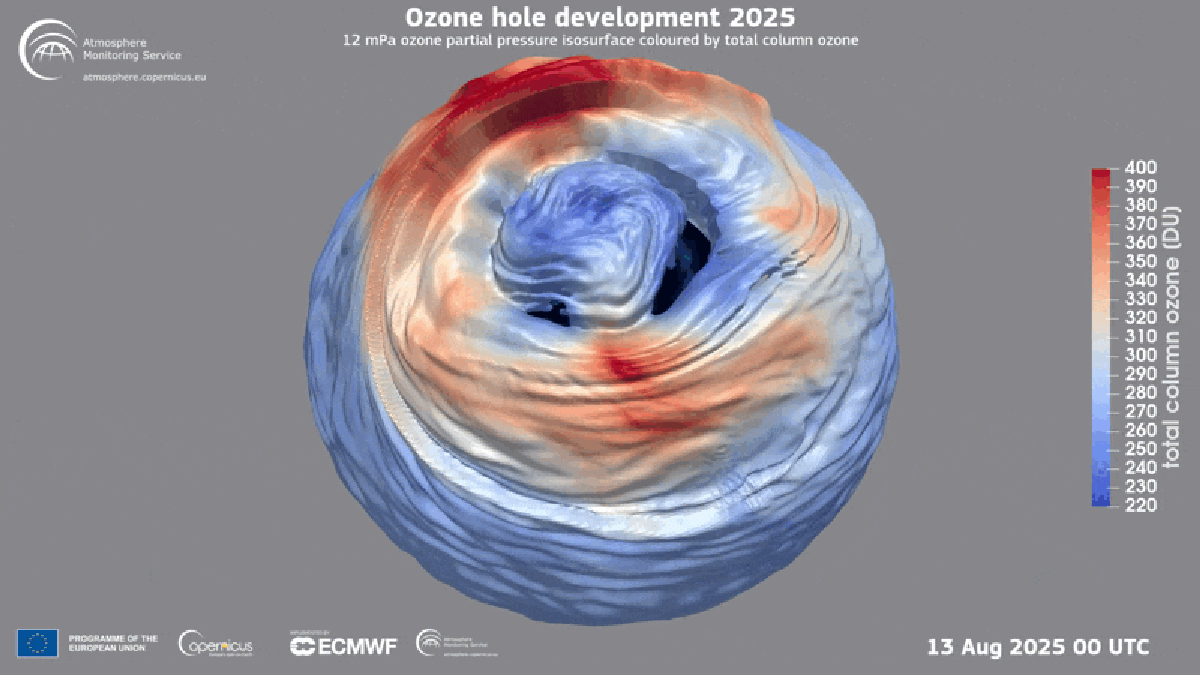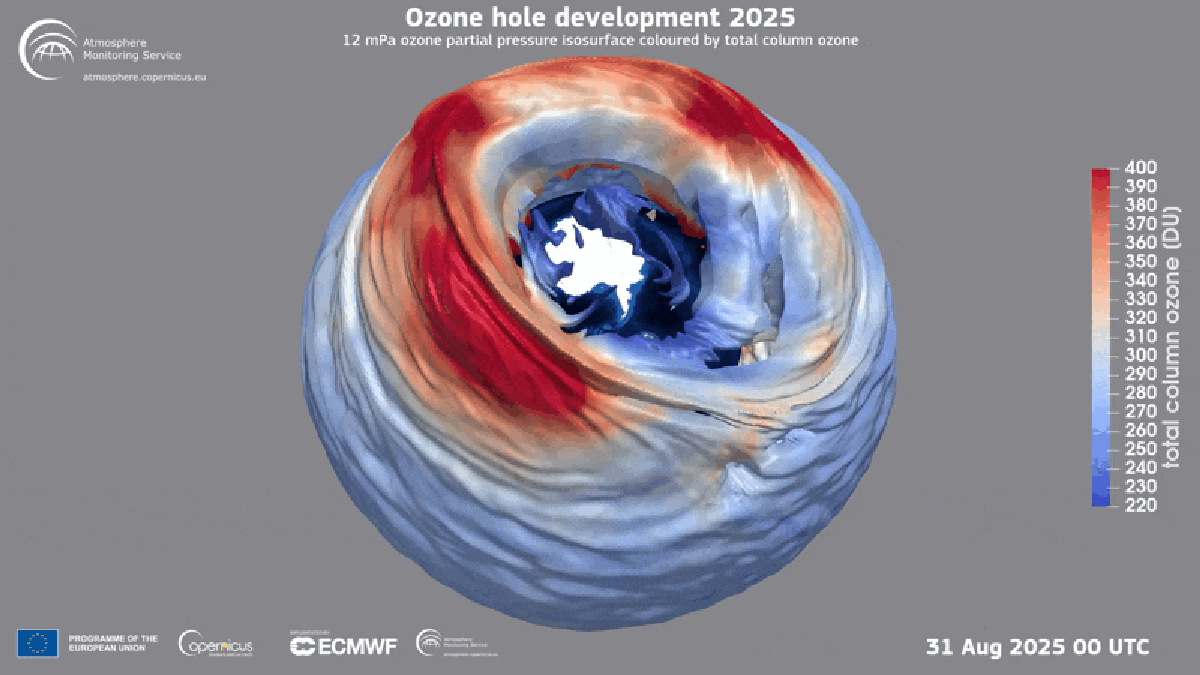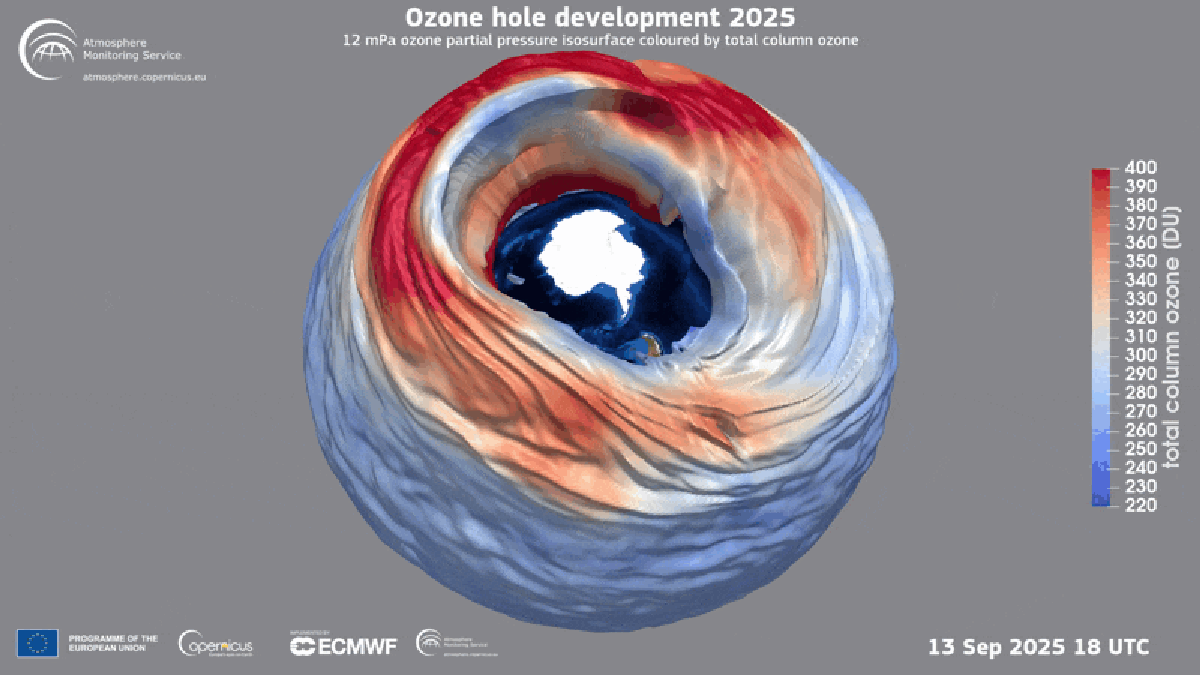
"Found between about nine and 19 miles above Earth's surface, the ozone layer is a broad region of the stratosphere where the molecule, which contains three oxygen atoms, is particularly concentrated. Here, ozone plays a vital role in blocking the sun's ultraviolet radiationessentially acting as a planetary sunscreen of a sort."
"In the 1980s, scientists realized that a massive hole was developing in the ozone layer over Antarctica every southern spring and then tied the observation back to earlier research that discovered that a group of chemicals called chlorofluorocarbons (CFCs) were able to eat away at atmospheric ozone. Nations came together to develop an agreement called the Montreal Protocol on Substances That Deplete the Ozone Layer, adopted in 1987, to stop the production of these chemicals."
"The Montreal Protocol is the best environmental agreement we've ever created, says Durwood Zaelke, an environmental policy expert at the University of California, Santa Barbara, and founder and president of the Institute of Governance & Sustainable Development, an organization that is focused on addressing short-lived but high-powered climate pollutants. These include hydrofluorocarbons (HFCs), which do not harm the ozone layer and replaced many CFCs as they were phased out. The agreement has garnered global signatories, several rounds of successful amendments and the near-total elimination of the chemicals that break down ozone."
Forty years after global policymakers began addressing the Antarctic ozone hole, the damage is continuing to heal, according to the World Meteorological Organization. The ozone layer exists roughly nine to 19 miles above Earth's surface in the stratosphere and concentrates ozone molecules made of three oxygen atoms. Ozone blocks the sun's ultraviolet radiation, acting as a planetary sunscreen. In the 1980s scientists linked a recurring Antarctic ozone hole to chlorofluorocarbons (CFCs) that destroy atmospheric ozone. Nations adopted the Montreal Protocol in 1987 to halt production of those chemicals, leading to near-total elimination of many ozone-depleting substances and enabling ongoing recovery. Hydrofluorocarbons (HFCs) replaced many CFCs and do not harm the ozone layer.
Read at www.scientificamerican.com
Unable to calculate read time
Collection
[
|
...
]