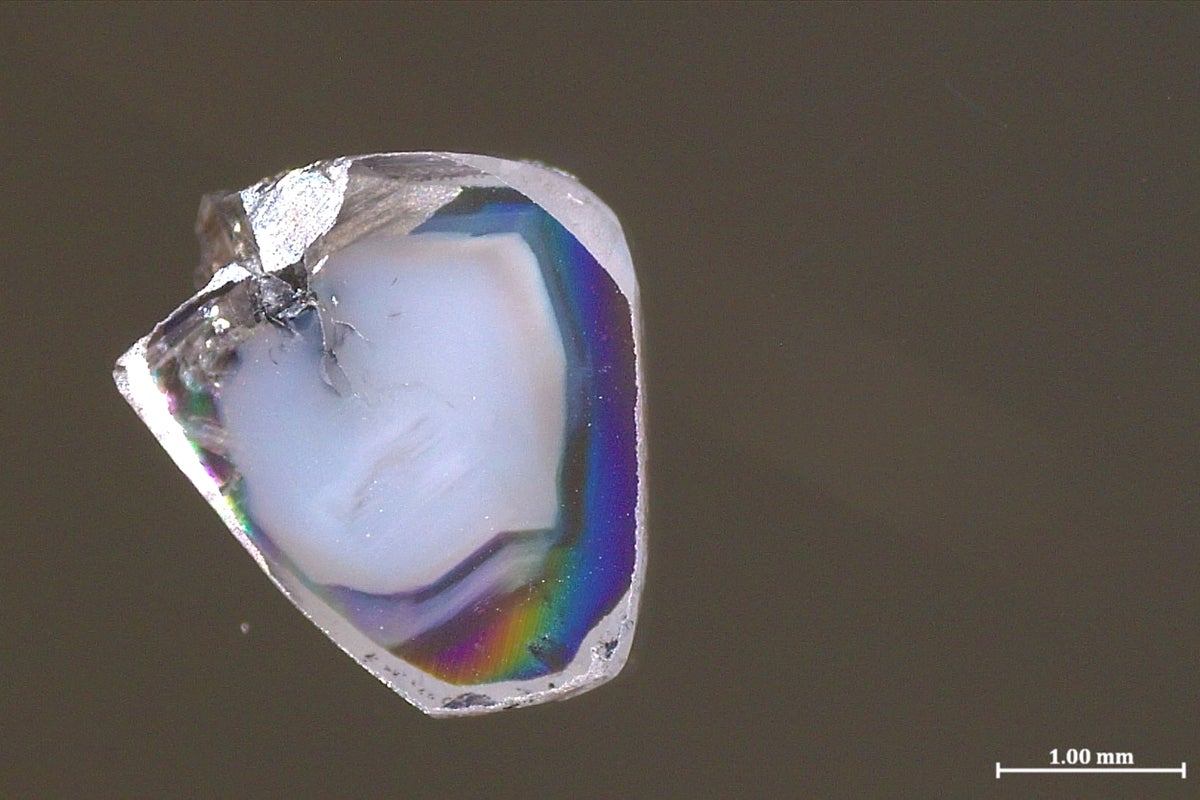
"A pair of diamonds that formed hundreds of kilometers deep in Earth's malleable mantle both contain specks of materials that form in completely opposing chemical environmentsa combination so unusual that researchers thought their coexistence was almost impossible. The substances' presence provides a window into the chemical goings-on of the mantle and the reactions that form diamonds. The two diamond samples were found in a South African mine. As with plenty of other precious gemstones, they contain what are called inclusionstiny bits of surrounding rocks captured as the diamonds form."
"The two new diamond samples each contain inclusions of carbonate minerals that are rich in oxygen atoms (a state known as oxidized) and oxygen-poor nickel alloys (a state known as reduced, in the parlance of chemistry). Much like how an acid and a base immediately react to form water and a salt, oxidized carbonate minerals and reduced metals don't coexist for long. Typically, diamond inclusions show just one or the other, so the presence of both perplexed Yaakov Weiss, a senior lecturer in Earth sciences at the Hebrew University of Jerusalem, and his colleaguesso much so that they initially put the samples aside for a year in confusion, he says."
Two diamonds formed hundreds of kilometers deep in Earth's mantle preserve microscopic inclusions of both oxygen-rich carbonate minerals and oxygen-poor nickel alloys. Oxidized carbonate phases and reduced metal phases normally react and rarely coexist, making this combination highly unexpected. Diamond inclusions remain essentially unchanged as diamonds transport them to the surface, providing intact samples of deep-mantle chemistry. The simultaneous presence of chemically opposing inclusions offers direct insight into mantle redox conditions and diamond-formation reactions and initially perplexed researchers who reexamined the samples to interpret the preserved mineral assemblages.
Read at www.scientificamerican.com
Unable to calculate read time
Collection
[
|
...
]