#clinical-trials
#clinical-trials
[ follow ]
#immunotherapy #semaglutide #alzheimers #mental-health #cancer-treatment #healthcare #rare-diseases #neuroscience
fromNature
3 weeks agoWhy cancer can come back years later - and how to stop it
When Lisa Dutton was declared free of breast cancer in 2017, she took a moment to celebrate with family and friends, even though she knew her cancer journey might not be over. As many as one-third of people whose breast tumours are cleared see the disease come back, sometimes decades later. Many other cancers are known to recur in the years following an initial treatment, some at much higher rates.
Medicine
fromwww.theguardian.com
1 month agoUse of traditional medicine in mainstream healthcare needs to be evidence-based | Letter
However, it is regrettable that an organisation such as the World Health Organization appears willing to promote the incorporation of traditional and other medicines into mainstream practice by leaning heavily on emotive language heritage, tradition, and the sharing of local resources rather than on clinical evidence. Then it appears to contradict itself by saying that it doesn't support it if there isn't robust and reliable evidence.
Yoga
fromLondon Business News | Londonlovesbusiness.com
1 month agoLondon trials: The business and patient impact - London Business News | Londonlovesbusiness.com
London's position as Europe's leading clinical research hub generates profound impacts extending far beyond laboratory walls and hospital wards. Hundreds of London trials running simultaneously create a sophisticated ecosystem connecting pharmaceutical companies, research institutions, healthcare providers, and most importantly, thousands of patients seeking access to innovative treatments whilst contributing to medical advancement. This ecosystem delivers tangible economic value whilst simultaneously addressing patient needs that conventional healthcare often cannot meet.
Miscellaneous
Medicine
fromwww.scientificamerican.com
2 months agoHalted NIH Clinical Trials List Reveals Slashed Treatments for Cancer, COVID and Minority Health
At least 383 NIH-funded clinical trials across diverse diseases were terminated since February, affecting about 74,000 participants and representing roughly 1 in 30 trials.
Cancer
fromwww.theguardian.com
2 months agoCancer Detectives: Finding the Cures review this vaccine documentary is so inspirational it'll make you weep
Advances in immune-system understanding enable development of vaccines to prevent cancers, with a 10-year pre-cancerous window offering intervention opportunities if clinical trials secure funding.
fromSFGATE
2 months agoBay Area biotech company declares bankruptcy, over $10 million in debts
The biotech company, which is headquartered in Milpitas, turned in a Chapter 7 filing, meaning that it seeks liquidation, rather than reorganization. The document is so sparse that it prompted a request from the court's deputy clerk for more information. But it depicts a company in dire straits: ASC Therapeutics estimates that it has between $100,000 and $500,000 in assets and between $10 million and $50 million in liabilities.
US news
fromwww.aljazeera.com
2 months agoCuban authorities battle wave of mosquito-borne illnesses
Cuba is battling a wave of mosquito-borne illnesses, with the country's top epidemiologist warning that nearly one-third of the population has been impacted, with large numbers of workers taken ill. On Thursday, fumigators armed with fogging machines probed alleys and crowded buildings in parts of the capital Havana, among the hardest hit by mosquito-borne viruses including dengue and chikungunya, authorities said.
Public health
Alternative medicine
fromAlternative Medicine Magazine
2 months agoUtiva Launches All-Natural Menopause Relief Supplement
Utiva Menopause Relief is a once-daily, non-hormonal, clinically supported plant-extract supplement that improves multiple common menopause symptoms including hot flashes and sleep.
fromNature
3 months agoPersonalized gene editing helped one baby: can it be rolled out widely?
The groundbreaking clinical trial, described on 31 October in the American Journal of Human Genetics, will deploy an offshoot of the CRISPR-Cas9 gene-editing technique called base editing, which allows scientists to make precise, single-letter changes to DNA sequences. The study is expected to begin next year, after its organizers spent months negotiating with US regulators over ways to simplify the convoluted path a gene-editing therapy normally has to take before it can enter trials.
Medicine
Women
fromFortune
3 months agoWomen's health is an 'economic blind spot.' Data is the key to reframing the conversation | Fortune
Women's health is human health and requires data-driven trust, equitable pharmaceutical development, women's leadership, and inclusion in digital platforms and clinical trials.
fromSocial Media Explorer
3 months agoA Doctor's Guide to Sharing Clinical Trial Breakthroughs on Social Media - Social Media Explorer
The announcement of a positive result from a major clinical trial is an incredibly exciting moment in the world of medicine. It's a validation of years of painstaking research, a potential new tool for doctors, and, most importantly, a powerful new source of hope for patients and their families. As a physician or a researcher, the desire to share this exciting news with the public is a natural one.
Medicine
fromsfist.com
3 months agoSam Altman-Backed SF Company Making Legal Ecstasy' Wins Approval to Conduct Clinical Trials
An SF-based biotech company is working on a safer, reformulated version of MDMA, better known as molly' or ecstasy,' and we're not tripping when we say they just won FDA approval to begin Phase 1 clinical trials. We have occasionally covered Bay Area medical research into MDMA, the street drug known as ecstasy or molly, and perhaps a little too enthusiastically at that.
Medicine
fromwww.scientificamerican.com
4 months agoHow Scientists Finally Found a Treatment that Slows Huntington's Disease
But last Wednesday was a day unlike any other. I cried with every single patient, Sung says. It just was this crazy feeling that, for the patients and families, almost can't feel real. That day the results of important phase 1/2 clinical trials had finally been released: an experimental gene therapy drug was the first treatment shown to slow the progression of Huntington's disease.
Medicine
fromZDNET
4 months agoAI agents are transforming the healthcare and life sciences industry
Life sciences leaders are increasingly adopting AI and AI agents to address growing industry disruption. This shift is occurring as the sector confronts new regulatory demands that strain compliance teams, increasingly complex clinical trials, and rising expectations from healthcare professionals. A recent Salesforce study revealed that life sciences leaders see AI as a powerful tool for navigating these challenges, with 94% expecting AI agents to be critical for scaling organizational capacity and strengthening operations.
Healthcare
fromwww.scientificamerican.com
4 months agoWhy Intermittent Fasting May Do More Harm Than Good
Recent headlines warning of concerns such as heart risks or danger to teenagers have put a new spotlight on a diet trend that has long been the popular epitome of a healthy lifestyle: intermittent fasting. Intermittent fasting's image has been deeply tarnishedand quite rightly so, says Stefan Kabisch, a physician at the endocrinology and metabolic medicine department at ChariteUniversity Medicine Berlin. The hype was never really backed up by good data in humans.
Science
fromwww.theguardian.com
4 months agoBritain is a terrible place' to sell medicines, says drug firm executive
We've still got the best universities, we've got some of the best scientists in the world, but it's not a good place to do the development work for medicines. It's an expensive place to operate, and it's a terrible place to sell medicines.
Medicine
fromNews Center
6 months ago'Dancing Molecules' Treatment Receives FDA Orphan Drug Designation - News Center
"Dancing molecules," the promising new treatment for acute spinal cord injuries developed at Northwestern University, has received Orphan Drug Designation from the U.S. Food and Drug Administration (FDA).
Science
fromwww.nature.com
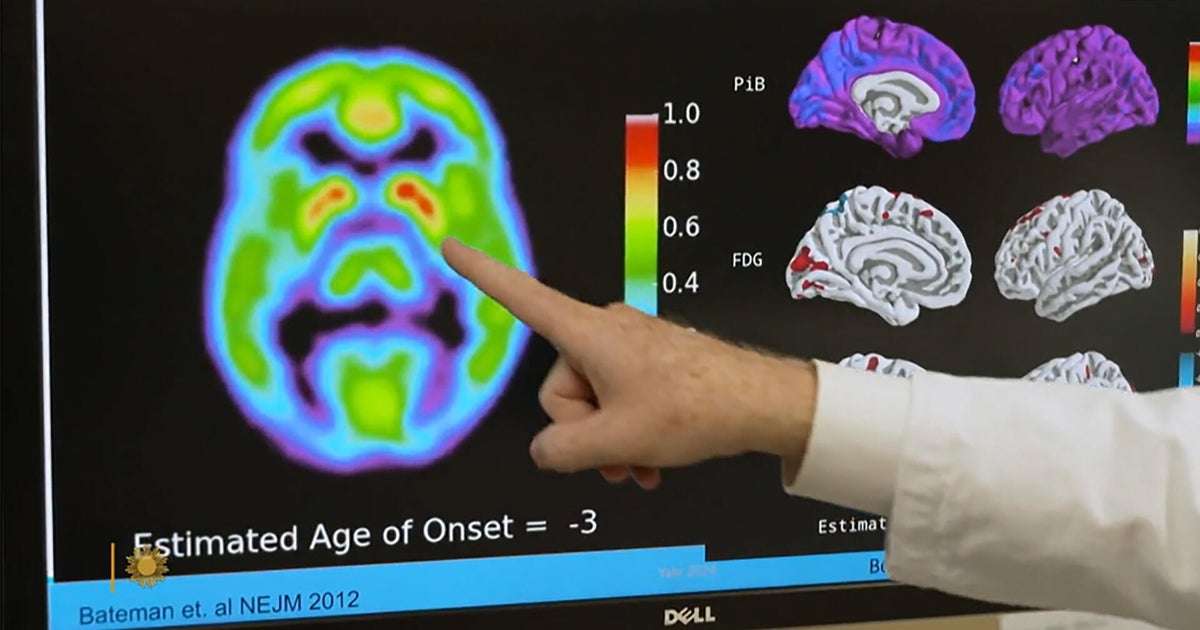
6 months agoNew Parkinson's Treatment Is like a Pacemaker for the Brain
Deep-brain stimulation involves inserting thin wires through two small holes in the skull into a region of the brain associated with movement. The hope is that by delivering electrical pulses to the region, the implant can normalize aberrant brain activity and reduce symptoms.
Healthcare
fromSilicon Canals
6 months agoBarcelona's Biorce raises 5M: CEO Pedro Coelho on tackling clinical trial delays with AI, US expansion, and hiring plans - Silicon Canals
Biorce is exactly the kind of pioneering company we're here to back. They're combining deep sector expertise with cutting-edge AI to solve what truly matters.
Healthcare
fromMiami Herald
6 months agoA paralyzed patient at UM just got Elon Musk's brain chip. Here are 5 takeaways
The Neuralink chip, known as the Link or Telepathy, was implanted by surgeons at the University of Miami Miller School of Medicine, marking the first such procedure at a Miami hospital. The chip allows RJ to wirelessly control a computer, as demonstrated by his ability to play video games using only his mind.
Artificial intelligence
fromwww.theguardian.com
6 months agoIs exercise really better than drugs for cancer remission? It's an appealing idea but it's misleading | Devi Sridhar
The study highlighted that combining structured exercise with chemotherapy can improve overall health outcomes in colon cancer recovery, showing that exercise should complement medical treatments.
Health
Startup companies
fromLondon Business News | Londonlovesbusiness.com
7 months agoProfessor Akhil Tripathi on why he's proud his company ExciteOSA is sending people to sleep - London Business News | Londonlovesbusiness.com
ExciteOSA aims to improve sleep for those with sleep apnoea and reduce snoring, addressing significant health issues.
Science
fromNature
8 months agoDaily briefing: NIH foreign-grant cuts could leave thousands without care worldwide
US funding cuts will halt hundreds of clinical trials abroad.
Sierra Leone is experiencing a severe mpox outbreak.
A new pen technology shows promise for detecting Parkinson’s disease.
Increasing bot traffic is threatening online academic resources.
Cancer
fromNews Center
8 months agoNew Scoring System Improves Colorectal Cancer Risk Prediction - News Center
A new scoring system may improve risk prediction and treatment guidance for colorectal cancer patients.
An analysis of nearly 38,000 patients led to the development of a standardized score based on seven variables.
[ Load more ]